Physically, a photon of light, or electromagnetic radiation, consists of a transverse electrical wave, propagating at a right angle to a magnetic wave, which is of equal amplitude, but out of phase by half a wavelength. In this way, the electromagnetic wave travels through space as the electric wave induces the perpendicular magnetic wave, and vice versa, transferring energy between the two waves, with each wave cycle. This allows the photon, to propagate through a vacuum without any transmission medium, such as is needed for sound (air) or water waves (water), for example.
Like other particles, quantum mechanics describes photons as having both wave-like and particle-like properties.
In a vacuum (and in the absence of a gravitational field), light always travels along straight lines, at precisely the same speed – approximately 300 million metres per second (3 x 108 ms-1). Mathematically, the speed of light is often represented by a lower-case ‘c’, as in Albert Einstein’s famous equation E = mc2. Einstein’s Special Theory of Relativity is based on the concept that no object with mass can travel faster than the speed of light.
Vision
If we can see an object, it is either because it is a source of light, or because it is being illuminated by a light source, and some of the photons that are striking it are being reflected or scattered from its surfaces in the right direction for them to enter our eyes.
The eye’s lens focuses light to form an image on the retina at the back of the eyeball. This image is then detected by photoreceptive cells, distributed across the surface of the retina, which convert the photons’ electromagnetic energy to an electrical signal that is transmitted via the optic nerve to the brain.
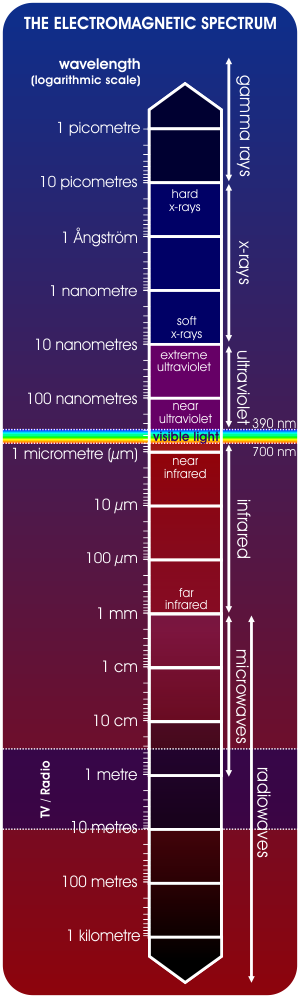
The Full Electromagnetic Spectrum
However, our eyes are only sensitive to photons within a certain energy range. The full electromagnetic spectrum stretches from gamma rays at the highest energies, through x-rays, ultraviolet light and the visible spectrum (from blue, through green, to red), to infrared light, microwaves and radio waves, at the lowest energies.
Since, (in simple ‘classical’ terms, at least), there is effectively no limit to the energy that can be possessed by an individual gamma-ray photon, and the energy of a radio-wavelength photon can be arbitrarily low, this electromagnetic spectrum can be considered to be effectively infinite in range.
Each photon of electromagnetic radiation has an associated wavelength and frequency, related to it’s energy via the equations:
frequency = energy / h
where h is Plank’s constant
and
wavelength = c / frequency
where c is the speed of light in a vacuum (approximately 3 x 108 metres per second)
Individual colours in the visible spectrum, as well as energies in other parts of the electromagnetic spectrum, therefore, each have a corresponding frequency and wavelength.
Photons in the radiowave range of the electromagnetic spectrum, at the low-energy end of the spectrum, have the longest wavelengths and lowest frequencies, whereas gamma rays have the highest frequencies and shortest wavelengths.
Light Sources
A source of light, or electromagnetic radiation, is an object that emits photons.
In the case of a light source such as the Sun or a lightbulb, enormous quantities of photons are emitted, travelling outwards in all directions. Photons in a laser beam, on the other hand, are all travelling in roughly the same direction, when they are emitted from the laser, with a very small angle of spread.
In quantum mechanics, a photon can be emitted or absorbed by a ‘quantum mechanical oscillator’, such as an electron – a negatively-charged particle – orbiting in the electomagnetic field surrounding the positively-charged nucleus of an atom.
Like the photon, and indeed all particles in the theory of quantum mechanics, the electron also has both wave-like and particle-like properties. The electron can only orbit the atomic nucleus in specific ‘quantised’ orbitals where, simply put, a whole number of wavelengths of the electron fit within each orbit.
The lowest energy orbit that the electron can take up, is closest to the atomic nucleus and is known as the ground state. The electron can jump to higher orbital levels corresponding to higher energy states – known as excited states – further out from the nucleus, by absorbing energy in the form of a photon. However, this can only occur if the photon’s energy happens to be the same as the difference between the two orbital energy levels.
Conversely, photons can also be emitted when an electron in an excited energy state (higher orbital level), drops down to a lower-energy orbital level. The emitted photon will then posses an energy corresponding to the difference between the energies of the two orbital levels.
A transition by the electron, in this way, directly between energy states, by emitting or absorbing a photon, without passing through intermediary states in between, is known as a ‘quantum leap’.
In fact, most sources of light operate via this mechanism, where a quantum oscillator, such as an electron orbiting an atomic nucleus, drops to a lower energy state, emitting a photon in the process.
A fire emits light because the electrons in the burning material are left in excited energy states following the chemical reactions of the fuel with oxygen from the air. The excited electrons emit photons of light and infrared radiation (heat) as they drop down to lower energy levels. These quantised energy levels, and hence the energies of the emitted photons, are different, depending on the substance that is burning, which is why different materials burn with different coloured flames.
In a traditional incandescent light bulb, electrons in the light bulb’s filament are pushed into higher energy levels by the flow of electricity through the filament. The electrons then drop back down to lower energy levels emitting photons of light and infrared radiation.
Starlight
The light from a star is first produced as high energy gamma-ray photons via nuclear fusion reactions in the star’s core. Rather than immediately radiating out into space, these photons are continuously re-absorbed, re-emitted and scattered, at different wavelengths. It can take tens or even hundreds of thousands of years for the energy from the core of the star to reach the surface. By the time it is emitted into space, the light will approximate a black-body radiation spectrum with its peak wavelength, and therefore colour, dependent on the star’s temperature (see quantum mechanics). Hotter stars emit more light towards the blue end of the spectrum, whereas cooler stars appear redder in colour.